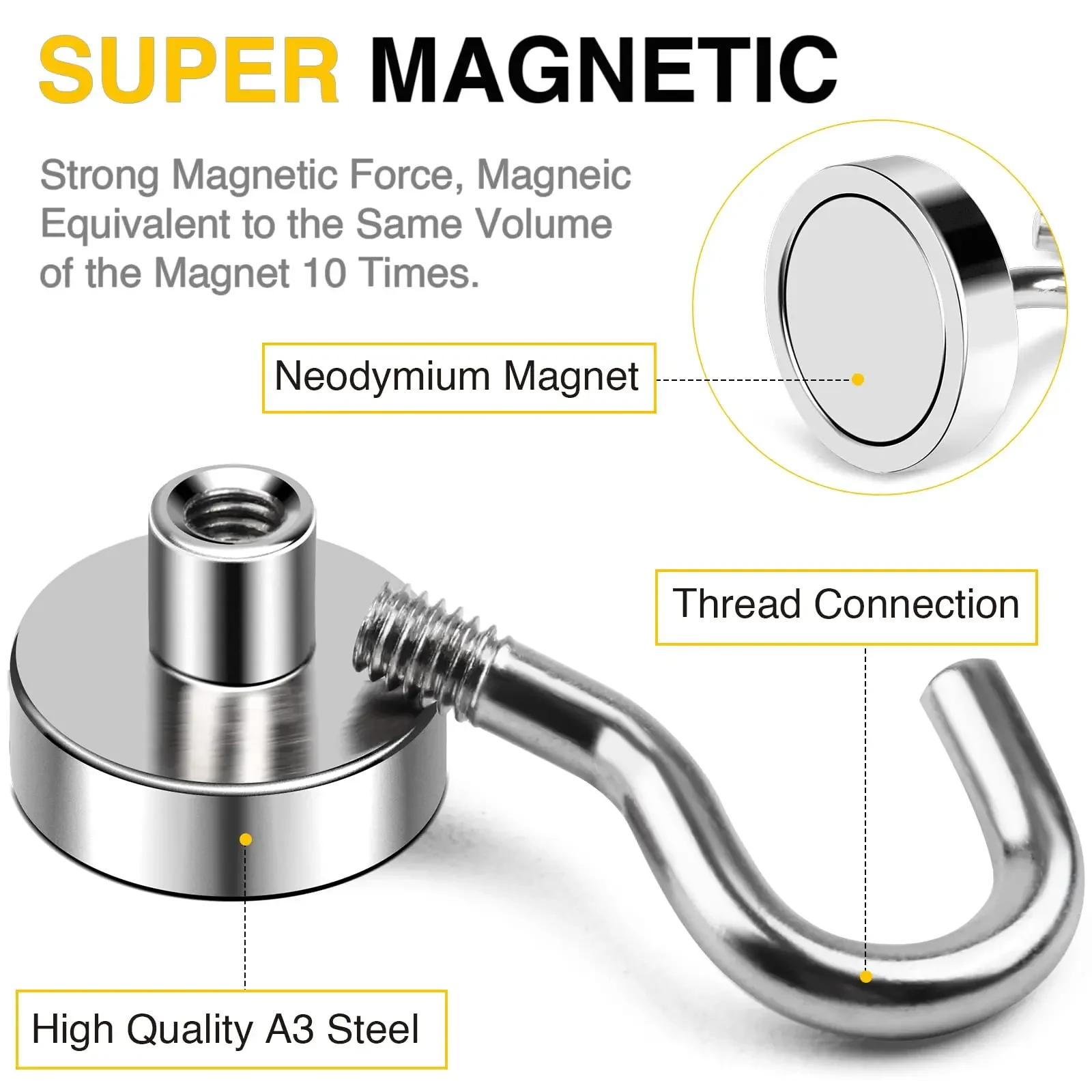
Rare earth купить от 80,00 руб.







Rare earth
The term "rare earth" describes a class of 17 chemical elements located in the bottom right corner of the periodic table. These elements are characterized by their rarity and scarcity, which is a consequence of their low abundance in nature.
Among these elements, dysprosium, erbium, dysprosium and dysprosium are some of the most valuable and sought after due to their applications in various industries. For instance, dysprosium is used in permanent magnets, while erbium is used in lasers and fluorescent lamps.
One of the main uses of rare earth elements is in the production of permanent magnets. These magnets are used in a variety of applications, such as motors, generators, and magnetic resonance imaging (MRI) machines. They also find use in magnetic recording media, such as hard disks and floppy disks.
Another important application of rare earths is in electronics. Dysprosium, for example, is used in flash memory devices, such as solid-state drives and hard drives. Erbium is also used in fiber optic communication systems and laser diodes.
Rare earth elements are also used in medicine. Dysprosium is an MRI contrast agent that helps doctors visualize tumors and other abnormalities in the body. Erbium-doped fiber optic probes are used to map blood vessels during surgery.
However, there are concerns about the sustainability of these elements. Many of them are mined from China, which has a monopoly on most of their production. This has led to concerns about environmental impact and potential resource depletion.
In order to address these concerns, it is important for governments and industry leaders to work together to develop alternative sources of rare earth materials. This could include using new technologies to extract elements from seawater or using biomass as a source of dysprosium.
Overall, rare earth elements are essential for our modern technological society. However, their scarcity and environmental impact warrant continued research into alternative sources and sustainable production methods.
The term "rare earth" describes a class of 17 chemical elements located in the bottom right corner of the periodic table. These elements are characterized by their rarity and scarcity, which is a consequence of their low abundance in nature.
Among these elements, dysprosium, erbium, dysprosium and dysprosium are some of the most valuable and sought after due to their applications in various industries. For instance, dysprosium is used in permanent magnets, while erbium is used in lasers and fluorescent lamps.
One of the main uses of rare earth elements is in the production of permanent magnets. These magnets are used in a variety of applications, such as motors, generators, and magnetic resonance imaging (MRI) machines. They also find use in magnetic recording media, such as hard disks and floppy disks.
Another important application of rare earths is in electronics. Dysprosium, for example, is used in flash memory devices, such as solid-state drives and hard drives. Erbium is also used in fiber optic communication systems and laser diodes.
Rare earth elements are also used in medicine. Dysprosium is an MRI contrast agent that helps doctors visualize tumors and other abnormalities in the body. Erbium-doped fiber optic probes are used to map blood vessels during surgery.
However, there are concerns about the sustainability of these elements. Many of them are mined from China, which has a monopoly on most of their production. This has led to concerns about environmental impact and potential resource depletion.
In order to address these concerns, it is important for governments and industry leaders to work together to develop alternative sources of rare earth materials. This could include using new technologies to extract elements from seawater or using biomass as a source of dysprosium.
Overall, rare earth elements are essential for our modern technological society. However, their scarcity and environmental impact warrant continued research into alternative sources and sustainable production methods.
Каталог Rare earth (art death)
Цены актуальны на 2025-09-13 05:52:35
Цена: 167 Руб. 1.85$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 594 Руб. 7.58$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цены актуальны на 2025-09-13 05:52:35
Цены актуальны на 2025-09-13 05:52:35
Цена: 1031 Руб. 10.06$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 274 Руб. 3.25$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 672 Руб. 6.23$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 1461 Руб. 17.32$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 89 Руб. 1.06$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цены актуальны на 2025-09-13 05:52:35
Цена: 2126 Руб. 25.2$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 719 Руб. 6.96$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 1432 Руб. 18.25$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 74 Руб. 0.95$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 1271 Руб. 16.09$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 1037 Руб. 11.34$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35
Цена: 1088 Руб. 12.9$
Бесплатная доставка
Цены актуальны на 2025-09-13 05:52:35